LostZombie
Transgirl Chemist
- Oct 10, 2025
- 131
Please note that there are some dangers to making SN. It's best to produce it under a fumehood or
outside with proper ventilation since the process involves production of toxic Nitrogen Dioxide.
Any references made have sources at the bottom of this guide
The first thing you will need to know is what is the chemical formula of SN, the other chemicals and maybe a bit of chem vocab
Mole: a unit that measures the amount of substance. It lets you convert between particles, mass, and volume by using molar mass (grams per mole) (shorthand for mole is mol)
SN = Sodium Nitrite = Na(NO2) = NaNO2
Sodium Nitrate = Na(NO3) = NaNO3
Calcium Sulfite = Ca(SO3) = CaSO3
Calcium Sulfate = Ca(SO4) = CaSO4
Methanol = CH4O = CH3OH
Will shorthand much of these just into
Nitrate = (NO3)
Nitrite = (NO2)
Sulfite = (SO3)
Sulfate = (SO4)
Methanol = MeOH
Required Materials:
Sodium Nitrate
Calcium Sulfite
Small Containers (metal, glass, or ceramic)
Small breaker like container (oven safe)
Oven (230C/450F/500K)
Coffee Filters
Funnel
Deionized Water (Type 2 pref)
Scale (in grams)
Rubber Gloves
Eye Protection (glasses will work)
Optional but recommended items:
(Swirlin/Erlen)meyer flask
Beakers
Concentrated Sulfuric Acid
Crystallization Dishes
Test Tubes
Mortar & Pestle
Lab Goggles
Fume mask (N95 mask will do fine)
Methanol (95%+)
Nitrate+Nitrite Testing Strips
The goal is to turn the sodium nitrate into sodium nitrite due to nitrate being much easier to acquire. The purer the better, and 99% pure Ca(SO3), and Na(NO3) can easily be found online. The layman's reason why Ca(SO3) is a great oxidizing agent for our purposes is because both the Ca reactant & product are insoluble which will make it easy to separate out the SN from the Ca(SO3/4).
Math Section:
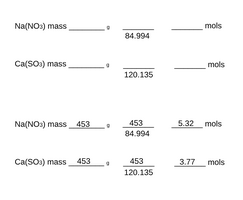
First convert the weight of both the substances you ordered into grams. I bought 1 pound bags of each so each will contain approximately 453 grams
Secondly you need to convert both substances into moles which tells you how many particles of each substance there are. For Na(NO3) the molar mass is 84.994 grams per mol so if you have 453g then you just divide it by 84.994 that will equal 5.3 moles of substance.
Now you need to repeat that again but this time with Ca(SO3) values. Which is 120.135 g/mol, so what I would do is 453 divided by 120.135 that equals 3.7
So we have 5.3 mols of NO3 and 3.7 mols of SO3. Luckily the reaction is 1:1 so all you need to know is whatever the lower mole number is your limiting factor, and how many mols of SN you will get, so in my case 3.7 max. The
I recommend doing 100 gram cooks at a time for the best yields which is 41.4g of Na(NO3) and 58.6g of Ca(SO3), and this is the ratio you must maintain 41.4% of the mass must be Na(NO3) and 58.6% must be Ca(SO3). This represents around half a mole for each substance.
Note: the is not the exact ratio, but it leaves the SO3 in excess so that all of the NO3 is reacted since SO3 is also insoluble
Here is a math sheet with my mass values as examples
That's about it for math!
Cooking Of The SN:
First of all, wear gloves, both substances will give you chemical burns, and be sure you have some eye protection on too.
You will now want to put both substances into bowl in equamolar amounts (pref SO3 to be in excess by a few grams) and crush them together until thoroughly mixed in a powered form (around 5 mins if it comes pre-powdered)
Next you want to transfer the mix preferably into a beaker or similar container, but want to be able to compress them both into until the mix feels solidly in place (like compressed flour).
If you are using a toaster oven, it's best to bring it outside for the cooking process. If you are unable to do this I would recommend using a bag or cover of some sort to prevent toxic fumes from escaping until you are able to take it outside.
With that you want to preheat your oven to 230C/450F/500K and take your mixture container, let it bake in the oven for 40 minutes, and be sure to keep an eye on it. (The goal is to heat the mixture as evenly as possible in a controlled temperature which is tricky to do with a burner or hot plate)
When time is about up, put on a mask, and eye protection when taking out the oven just in case since there will most likely be orange fumes coming out of the beaker, and that is toxic Nitrogen Dioxide (N2O), I would recommend taking it outside, or putting it next to a window to let the fumes escape.
(N2O colors in various concentrations)
So the cooking part is done, now after it has cooled down you want to take your distilled water and put some in the beaker and break up the compound since I will have fused together (like soft sandstone), just use a spoon & deionized water and you will be fine.
You can break down the compounds and add it to another bowl where you break up all the clumps until it has a soupy consistency.
Now take your funnel and coffee filter and you pour your mixture through it and water will pass through into your swirlinmeyer flash while a mushy substance will remain, and be unable to pass through the filter. Pour some extra water after all of the compound mix has been filtered to ensure you get the most SN you can out of it. Do not pour more than 100 ml at a time to extract the extra SN as it is possible that a small amount of (SO3/4) could dissolve and contaminate your SN.
Once you have finished the filtering process you then pour your water+SN mix into a crystallization dish, or a flat dish that can hold the water.
Now put it in the oven at 95C/200F/370K until all the water has evaporated (Don't go over 95C)
Evaporating the solution will leave you with white, powder/crystals which are mostly pure SN. It is important to note that you will still have a small amount of nitrates in the resulting crystals which is inevitable after a cook. Take out your product and weigh out your SN to see how much you have. (You will likely not have enough to CTB on your first cook so be ready to cook 2-3 batches)
Purifying SN With Methanol:
After all of this I would recommend doing some extra steps to ensure the purity of your SN
Na(NO2) soluble in methanol (4.4 g/100 mL) at 20C so for 20 grams of SN you would want 475 ml~ of MeOH and put through a coffee filter that can give you give you a guarantee of a minimum 75% purity after boiling away the MeOH 65C/150F/340K, but more likely 95%+ if there was excess Ca(SO3) in the cook (assuming that NO3 is the only contaminant) due to general loss of mass often in experiments. (I got 98% pure from my cook+purification)
You MUST do the boiling of the MeOH outside, with either a hot plate or any other sort of heating device outside to prevent methanol from condensing inside.
(SN Refinement setup)
Testing SN Purity:
Concentrated Sulfuric acid (18.4 Molar/98.3%) will spontaneously react with SN to create N2O which will appear as orange gas, and if your SN has been purified it will likely be pure enough for further purity testing. Use a small amount of SN in a test tube and add a few drops of sulfuric acid, and close up the tube to watch the reaction.
(Acid+SN Rxn)
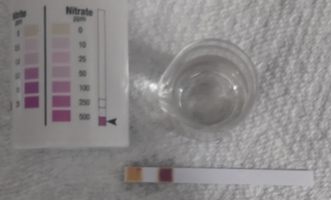
Nitrate+Nitrite testing strips are relatively cheap to find online, I would recommend using Vizzy's guide. However, to test the ratio of NO3 to NO2 (purity) test how much NO3 there is vs NO2. For example if the NO3 has 12 ppm, and the NO2 has 24 ppm its a 1:2 ratio a 66% pure batch.
(Control Test)
Dos and Don'ts:
Do not leave an open flame anywhere near the substances, since in this lab most of the substances are flammable, if not explosive.
Do not put any substances in a microwave, this may cause autoignition of the substances.
Do not touch any of the substances as they will be an irritant to your body, and could cause serious rashes.
Do not heat any of the substances over 300C/575F/575K after that point you risk autoignition of the chemicals, and they could explode, not just be set on fire.
Do not leave any substances in hot/humid environments as that could cause possible decommission of the substances. (Just keep them inside, and in a dark area)
Do use paper towels, on the countertop you decide to use to make cleanup easier.
Do wear protective gear that prevents chemical burns, eye irritation, and inhalation of toxins at all times when handing any of these chemicals.
Do these activities outside if it is not humid, foggy, rainy, and not over (35C/100F/305K) to prevent any buildup of any potential toxins. However if one of the following is not possible, or it would arouse suspicion to do it outside, then cook in your kitchen.
That's It!
That's all you need to know to make your very own SN from scratch!
Other Notes:
In theory this method could also work with K(NO3) to KN in theory. K(NO3) would also be harder to find due to the very small tiny irrelevant fact it can be used to make gunpowder. It would in theory work the same since it is also a group one metal. However the purification methods will likely need to change.
I would also like to note that my methods are not perfect, and if you have difficulty making some of this stuff I would recommend asking someone who knows what they are doing to answer your questions (do not ask ChatGPT it will give you bad advice or any AI)
This purification method could be used on curing salts to extract nitrite since NaCl has only (1.1g/100ml) while SN has (4.4g/100ml) at 25C, but that would result in a 80% purity SN so anyone who wants to figure out a method that can further refine SN for curing salts.
For those wondering, you could adjust the cook time if you wanted a more pure product, although it would require some testing to find where the sweet spot is, (for my 100g cook 40 mins at 230C was perfect for me) as to not decompose the materials into pure N2O, or having too little nitrite.
Sources:
Basic Solubility Rules
Periodic Table
Oxidizing and Reducing
Vizzy's SN guide
SN Solubility
Sodium Nitrite
Ml To Grams Conversions
Nitrogen Dioxide - Wikipedia
Sodium nitrite - Sciencemadness Wiki
sodium nitrite production, UPR, ecoinvent 3.6, Undefined | GLAD
Autoignition Temperature Data
Sulfuric Acid - Wikipedia
Sodium Nitrate | NaNO3 | CID 24268 - PubChem
Original Video Of SN Making
Special Thanks!
Vizzy, for his extensive guide on SN it was so well produced, what a guy!
Hiro-Uchiha, they proofread my work to make sure it all looks legit, and checked my spelling.
Apoptosis (yt) for proving a solid SN formula, allowing my project to get off the ground.
My HS chemistry teacher for teaching me about the great subject of chemistry!
Everyone on SaSu, y'all have been nothing but good to me even when the world rejects me!
outside with proper ventilation since the process involves production of toxic Nitrogen Dioxide.
Any references made have sources at the bottom of this guide
The first thing you will need to know is what is the chemical formula of SN, the other chemicals and maybe a bit of chem vocab
Mole: a unit that measures the amount of substance. It lets you convert between particles, mass, and volume by using molar mass (grams per mole) (shorthand for mole is mol)
SN = Sodium Nitrite = Na(NO2) = NaNO2
Sodium Nitrate = Na(NO3) = NaNO3
Calcium Sulfite = Ca(SO3) = CaSO3
Calcium Sulfate = Ca(SO4) = CaSO4
Methanol = CH4O = CH3OH
Will shorthand much of these just into
Nitrate = (NO3)
Nitrite = (NO2)
Sulfite = (SO3)
Sulfate = (SO4)
Methanol = MeOH
Required Materials:
Sodium Nitrate
Calcium Sulfite
Small Containers (metal, glass, or ceramic)
Small breaker like container (oven safe)
Oven (230C/450F/500K)
Coffee Filters
Funnel
Deionized Water (Type 2 pref)
Scale (in grams)
Rubber Gloves
Eye Protection (glasses will work)
Optional but recommended items:
(Swirlin/Erlen)meyer flask
Beakers
Concentrated Sulfuric Acid
Crystallization Dishes
Test Tubes
Mortar & Pestle
Lab Goggles
Fume mask (N95 mask will do fine)
Methanol (95%+)
Nitrate+Nitrite Testing Strips
The goal is to turn the sodium nitrate into sodium nitrite due to nitrate being much easier to acquire. The purer the better, and 99% pure Ca(SO3), and Na(NO3) can easily be found online. The layman's reason why Ca(SO3) is a great oxidizing agent for our purposes is because both the Ca reactant & product are insoluble which will make it easy to separate out the SN from the Ca(SO3/4).
Math Section:
First convert the weight of both the substances you ordered into grams. I bought 1 pound bags of each so each will contain approximately 453 grams
Secondly you need to convert both substances into moles which tells you how many particles of each substance there are. For Na(NO3) the molar mass is 84.994 grams per mol so if you have 453g then you just divide it by 84.994 that will equal 5.3 moles of substance.
Now you need to repeat that again but this time with Ca(SO3) values. Which is 120.135 g/mol, so what I would do is 453 divided by 120.135 that equals 3.7
So we have 5.3 mols of NO3 and 3.7 mols of SO3. Luckily the reaction is 1:1 so all you need to know is whatever the lower mole number is your limiting factor, and how many mols of SN you will get, so in my case 3.7 max. The
I recommend doing 100 gram cooks at a time for the best yields which is 41.4g of Na(NO3) and 58.6g of Ca(SO3), and this is the ratio you must maintain 41.4% of the mass must be Na(NO3) and 58.6% must be Ca(SO3). This represents around half a mole for each substance.
Note: the is not the exact ratio, but it leaves the SO3 in excess so that all of the NO3 is reacted since SO3 is also insoluble
Here is a math sheet with my mass values as examples
That's about it for math!
Cooking Of The SN:
First of all, wear gloves, both substances will give you chemical burns, and be sure you have some eye protection on too.
You will now want to put both substances into bowl in equamolar amounts (pref SO3 to be in excess by a few grams) and crush them together until thoroughly mixed in a powered form (around 5 mins if it comes pre-powdered)
Next you want to transfer the mix preferably into a beaker or similar container, but want to be able to compress them both into until the mix feels solidly in place (like compressed flour).
If you are using a toaster oven, it's best to bring it outside for the cooking process. If you are unable to do this I would recommend using a bag or cover of some sort to prevent toxic fumes from escaping until you are able to take it outside.
With that you want to preheat your oven to 230C/450F/500K and take your mixture container, let it bake in the oven for 40 minutes, and be sure to keep an eye on it. (The goal is to heat the mixture as evenly as possible in a controlled temperature which is tricky to do with a burner or hot plate)
When time is about up, put on a mask, and eye protection when taking out the oven just in case since there will most likely be orange fumes coming out of the beaker, and that is toxic Nitrogen Dioxide (N2O), I would recommend taking it outside, or putting it next to a window to let the fumes escape.
(N2O colors in various concentrations)
So the cooking part is done, now after it has cooled down you want to take your distilled water and put some in the beaker and break up the compound since I will have fused together (like soft sandstone), just use a spoon & deionized water and you will be fine.
You can break down the compounds and add it to another bowl where you break up all the clumps until it has a soupy consistency.
Now take your funnel and coffee filter and you pour your mixture through it and water will pass through into your swirlinmeyer flash while a mushy substance will remain, and be unable to pass through the filter. Pour some extra water after all of the compound mix has been filtered to ensure you get the most SN you can out of it. Do not pour more than 100 ml at a time to extract the extra SN as it is possible that a small amount of (SO3/4) could dissolve and contaminate your SN.
Once you have finished the filtering process you then pour your water+SN mix into a crystallization dish, or a flat dish that can hold the water.
Now put it in the oven at 95C/200F/370K until all the water has evaporated (Don't go over 95C)
Evaporating the solution will leave you with white, powder/crystals which are mostly pure SN. It is important to note that you will still have a small amount of nitrates in the resulting crystals which is inevitable after a cook. Take out your product and weigh out your SN to see how much you have. (You will likely not have enough to CTB on your first cook so be ready to cook 2-3 batches)
Purifying SN With Methanol:
After all of this I would recommend doing some extra steps to ensure the purity of your SN
Na(NO2) soluble in methanol (4.4 g/100 mL) at 20C so for 20 grams of SN you would want 475 ml~ of MeOH and put through a coffee filter that can give you give you a guarantee of a minimum 75% purity after boiling away the MeOH 65C/150F/340K, but more likely 95%+ if there was excess Ca(SO3) in the cook (assuming that NO3 is the only contaminant) due to general loss of mass often in experiments. (I got 98% pure from my cook+purification)
You MUST do the boiling of the MeOH outside, with either a hot plate or any other sort of heating device outside to prevent methanol from condensing inside.
(SN Refinement setup)
Testing SN Purity:
Concentrated Sulfuric acid (18.4 Molar/98.3%) will spontaneously react with SN to create N2O which will appear as orange gas, and if your SN has been purified it will likely be pure enough for further purity testing. Use a small amount of SN in a test tube and add a few drops of sulfuric acid, and close up the tube to watch the reaction.
(Acid+SN Rxn)
Nitrate+Nitrite testing strips are relatively cheap to find online, I would recommend using Vizzy's guide. However, to test the ratio of NO3 to NO2 (purity) test how much NO3 there is vs NO2. For example if the NO3 has 12 ppm, and the NO2 has 24 ppm its a 1:2 ratio a 66% pure batch.
(Control Test)
Dos and Don'ts:
Do not leave an open flame anywhere near the substances, since in this lab most of the substances are flammable, if not explosive.
Do not put any substances in a microwave, this may cause autoignition of the substances.
Do not touch any of the substances as they will be an irritant to your body, and could cause serious rashes.
Do not heat any of the substances over 300C/575F/575K after that point you risk autoignition of the chemicals, and they could explode, not just be set on fire.
Do not leave any substances in hot/humid environments as that could cause possible decommission of the substances. (Just keep them inside, and in a dark area)
Do use paper towels, on the countertop you decide to use to make cleanup easier.
Do wear protective gear that prevents chemical burns, eye irritation, and inhalation of toxins at all times when handing any of these chemicals.
Do these activities outside if it is not humid, foggy, rainy, and not over (35C/100F/305K) to prevent any buildup of any potential toxins. However if one of the following is not possible, or it would arouse suspicion to do it outside, then cook in your kitchen.
That's It!
That's all you need to know to make your very own SN from scratch!
Other Notes:
In theory this method could also work with K(NO3) to KN in theory. K(NO3) would also be harder to find due to the very small tiny irrelevant fact it can be used to make gunpowder. It would in theory work the same since it is also a group one metal. However the purification methods will likely need to change.
I would also like to note that my methods are not perfect, and if you have difficulty making some of this stuff I would recommend asking someone who knows what they are doing to answer your questions (do not ask ChatGPT it will give you bad advice or any AI)
This purification method could be used on curing salts to extract nitrite since NaCl has only (1.1g/100ml) while SN has (4.4g/100ml) at 25C, but that would result in a 80% purity SN so anyone who wants to figure out a method that can further refine SN for curing salts.
For those wondering, you could adjust the cook time if you wanted a more pure product, although it would require some testing to find where the sweet spot is, (for my 100g cook 40 mins at 230C was perfect for me) as to not decompose the materials into pure N2O, or having too little nitrite.
Sources:
Basic Solubility Rules
Periodic Table
Oxidizing and Reducing
Vizzy's SN guide
SN Solubility
Sodium Nitrite
Ml To Grams Conversions
Nitrogen Dioxide - Wikipedia
Sodium nitrite - Sciencemadness Wiki
sodium nitrite production, UPR, ecoinvent 3.6, Undefined | GLAD
Autoignition Temperature Data
Sulfuric Acid - Wikipedia
Sodium Nitrate | NaNO3 | CID 24268 - PubChem
Original Video Of SN Making
Special Thanks!
Vizzy, for his extensive guide on SN it was so well produced, what a guy!
Hiro-Uchiha, they proofread my work to make sure it all looks legit, and checked my spelling.
Apoptosis (yt) for proving a solid SN formula, allowing my project to get off the ground.
My HS chemistry teacher for teaching me about the great subject of chemistry!
Everyone on SaSu, y'all have been nothing but good to me even when the world rejects me!
Last edited: