Q
qwe78978213
Member
- Mar 19, 2024
- 9
1.Nitrite (NO2) and SN(NaNO2) are not the same substance. While they both contain nitrite ions (NO2^-), sodium nitrite also contains sodium ions (Na^+). So, they are different compounds.
However, many people try to test SN(NaNO2) using Nitrite (NO2) test kits.

2."NO2" and "NaNO2" are different words from the beginning, and even Wikipedia pages are divided into two. If they were the same substance, there should be only one page.
 en.wikipedia.org
en.wikipedia.org
 en.wikipedia.org
en.wikipedia.org
3.The CAS Registry Numbers, which are identification numbers for chemical substances, are also different.
Nitrite (NO2) CAS Number:14797-65-0
Sodium nitrite (NaNO2) CAS Number:7632-00-0
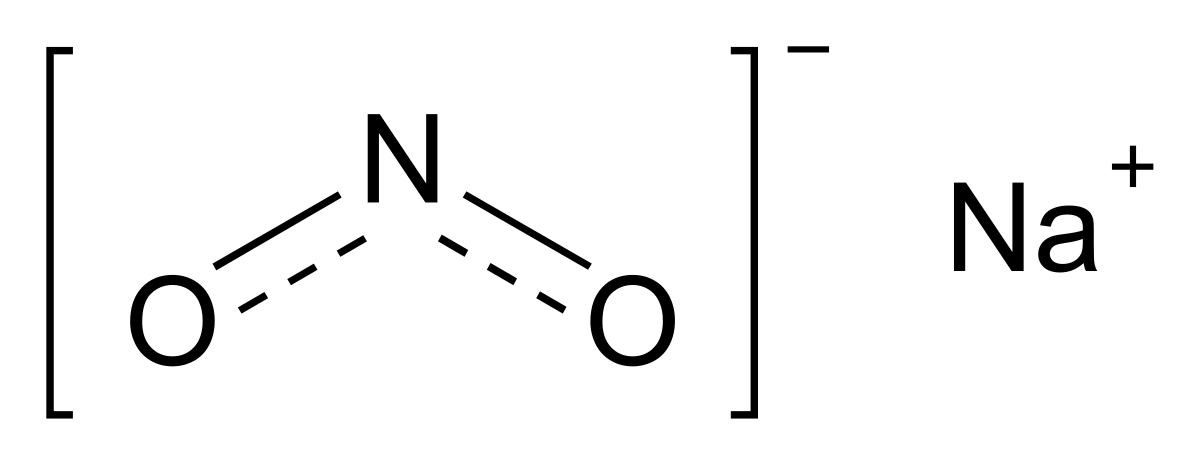
4.It's difficult to say that nitrite (NO2) and sodium nitrite (NaNO2) have the same chemical structure.



5.Testing sodium nitrite (NaNO2) using tools designed for testing nitrite (NO2) appears to be prone to error, and according to ChatGPT's response, it is as follows:
cannot directly test sodium nitrite (NaNO2) using a kit or reagent designed for testing nitrite (NO2). This is because sodium nitrite is a specific compound that contains nitrite ions along with sodium ions, and the test kits or reagents are typically designed to react with the nitrite ion itself. The presence of sodium ions in sodium nitrite may interfere with the reaction or give false results.


6.If some individuals were unable to properly test sodium nitrite (NaNO2) using nitrite (NO2) specific aquarium or drinking water test kits, it wouldn't be surprising.
However, many people try to test SN(NaNO2) using Nitrite (NO2) test kits.

2."NO2" and "NaNO2" are different words from the beginning, and even Wikipedia pages are divided into two. If they were the same substance, there should be only one page.

Sodium nitrite - Wikipedia
Nitrite - Wikipedia
3.The CAS Registry Numbers, which are identification numbers for chemical substances, are also different.
Nitrite (NO2) CAS Number:14797-65-0
Sodium nitrite (NaNO2) CAS Number:7632-00-0
4.It's difficult to say that nitrite (NO2) and sodium nitrite (NaNO2) have the same chemical structure.



5.Testing sodium nitrite (NaNO2) using tools designed for testing nitrite (NO2) appears to be prone to error, and according to ChatGPT's response, it is as follows:
cannot directly test sodium nitrite (NaNO2) using a kit or reagent designed for testing nitrite (NO2). This is because sodium nitrite is a specific compound that contains nitrite ions along with sodium ions, and the test kits or reagents are typically designed to react with the nitrite ion itself. The presence of sodium ions in sodium nitrite may interfere with the reaction or give false results.


6.If some individuals were unable to properly test sodium nitrite (NaNO2) using nitrite (NO2) specific aquarium or drinking water test kits, it wouldn't be surprising.
Attachments
Last edited: